New York- and Australia- based, Smileyscope Holding Inc (Smileyscope™) is a global pioneer in virtual and augmented reality (VR/AR) digital therapeutics. The company merges evidence-based medicine with state-of-the-art technology, providing ground-breaking digital treatments that help patients manage pain, anxiety, and mental health.
Smileyscope is pleased to announce its flagship VR needle experience has been granted U.S. Food and Drug Administration (FDA) Safer Technologies Program (STeP) entrance. This program is intended to expedite market access for products which are “reasonably expected to significantly improve the safety of currently available treatments or diagnostics” in addition to fulfilling other FDA criteria(1). Smileyscope’s proposed indications are for use in patients aged 4 years and older, intended to reduce and/or manage pain, anxiety and distress associated with needle procedures.
Chief Executive Officer Dr Evelyn Chan commented “Safer Technologies Program entrance is a key milestone in Smileyscope’s U.S. regulatory journey and highlights our position as the pre-eminent VR in acute healthcare.”
This approval pathway is a collaborative program which FDA intends to incorporate features such as interactive and timely communications, early engagement on Data Development Plans, sprint discussions, and senior management engagement.
Safer Technologies Program entrance builds on Smileyscope’s formal regulatory approvals as a medical device in three countries: Australia, the European Union and the United Kingdom, as well as recent guideline recommendation in the globally influential Infusion Therapy Standards of Practice, 8th edition.
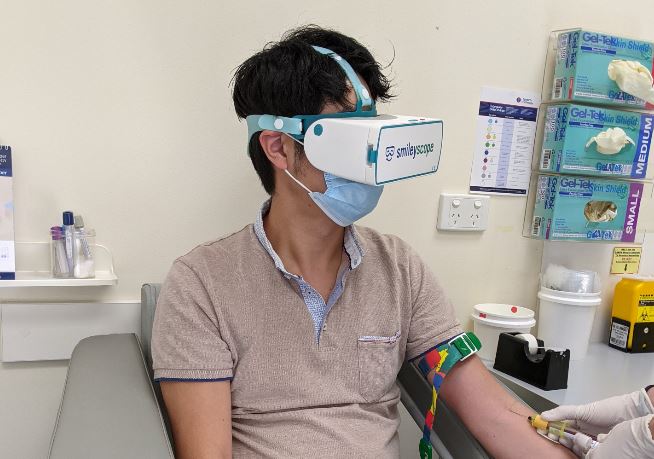
Smileyscope’s flagship VR needle experience was tested in two large randomized clinical studies, published in the prestigious peer-reviewed The Journal of Pediatrics. These trials found reductions in pain, anxiety, distress and the need to restrain children(2). Patients go on a relaxing underwater adventure while the procedure occurs in the real world.
Smileyscope’s flagship needle experience includes over 20 best practice techniques including the company’s patented VR innovations. The company’s VR headset is the world’s only VR headset that was constructed from the ground up with healthcare in mind.
Chief Medical Officer Dr Paul Leong remarked “As physicians, Dr Chan and I saw the problem of pain and anxiety first-hand. We have created a unique and powerful product which transforms needle pain and anxiety. We have received over 30 international awards spanning evidence-based medicine, design and business, and this adds to the recognition of our work.”
References
1. U.S. FDA. Safer Technologies Program for Medical Devices Guidance for Industry and
Food and Drug Administration Staff, January 6, 2021. Available from https://www.fda.gov/media/130815/download
2. Chan, E., … Leong, P. (2019). Virtual Reality for Pediatric Needle Procedural Pain: Two Randomized Clinical Trials. The Journal of Pediatrics, 209, 160–167. https://doi.org/10.1016/j.jpeds.2019.02.034
About Smileyscope
Smileyscope™ is a leading pioneer in virtual and augmented reality (VR/AR) therapeutics focused on managing pain, anxiety, and mental health for patients. The company has patented fundamental innovations in VR/AR delivery and has the only VR headset worldwide that was constructed from the ground up with healthcare in mind. The company currently has multiple clinical trials underway and is rapidly expanding.
511 The Avenue of the Americas, #4075
New York, NY 10011
1888 300 7117























