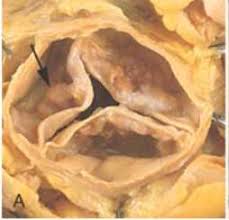
Valvublator, a development stage medical device company with a heart valve decalcification and regeneration platform technology, unveiled its Valvublator II 2nd generation product at Innovation Showcases this week in San Francisco and Palo Alto. The platform features the only system intended to not only decalcify diseased heart valves but also to regenerate them and prevent them from re-calcifying over the long term. The product is designed with intent to help people keep their own heart valves instead of getting a cow, pig, steel or plastic artificial implant,
Animation Video Link to the Valvublator II design https://vimeo.com/361935548/5ba8932782
"Natural heart valves undeniably out perform any tissue or mechanical artificial heart valve. Both open chest and percutaneous heart valve replacements have associated risks" said Mark Cunningham, MD, a cardiac surgeon at USC Keck Medical Center and Chief Scientific Advisor to Valvublator.
The Valvublator II design is a unique system platform addressing heart valve decalcification, regeneration and prevention of re-calcification.
- Simple single metal basket tip on end of a percutaneous catheter. Easy to place in position.
- Step 1 = ultrasonic energy to break up calcification.
- Step 2 = delivery of precise bioelectric signaling sequences to release stem cell homing proteins and other regeneration promoting factors.
- Step 3 = at home application of simple electrodes on thigh muscle 1X a month for about 15 minutes to release circulating Klotho to prevent re-calcification.
- Step 4 = periodic injection of Elastrin Therapeutics Inc. targeted nanoparticles to support de-calcification and prevent re-calcification’ www.elastrin.com
In the United States, surgeons perform over 110,000 heart valve operations each year. Nearly all of these operations are done to repair or replace the mitral or aortic valves.
“This simple to use system may be another choice for patients suffering of heart valve disease. In the future we hope this product may be used to clean up and regenerate heart valves BEFORE they are so advanced in disease that a replacement is deemed necessary." stated Dr. Brett Burton Vice President of R&D at Valvublator and lead engineer working on the project. “This simple procedure could address a significant portion of patients who otherwise would be referred to open-heart or transcatheter based heart valve replacement, allowing them to keep their own heart valve instead."
Valvublator is in the early stages of gathering pre-clinical data with intent to soon file a pre-submission with the FDA to define its clinical pathway leading to a PMA. The company also is defining its regulatory and clinical strategy for OUS clinical studies. The Valvublator device is intended to always be used with commercially available FDA or CE Mark cleared cerebral/emboli protection devices to capture or deflect any stray fragments.
About Valvublator:
Valvublator, www.valvublator.com, is a development-stage medical device company developing an innovative platform to permit patients to keep their own heart valves, decalcified and regenerated, instead of getting cow, pig, plastic or steel artificial implants. Valvublator is incubating within the Leonhardt's Launchpads accelerator labs in Los Angeles, Utah, Pittsburgh and Brazil. The Valvublator II design was developed in collaboration with REV1 Engineering of Murrieta, California - https://rev1engineering.com
About Leonhardt's Launchpads Innovation Showcases and DEMO DAYS Organ Regeneration 2019:
Leonhardt's Launchpads will showcase 30 startups from its 2019 portfolio class https://leonhardtventures.com/development-pipeline/ all focused on organ regeneration and recovery including Valvublator:
Friday, September 27th, 4 to 6pm @ WeWork, 3101 Park Blvd.,Palo Alto - for tickets click here https://www.eventbrite.com/e/leonhardts-launchpads-demo-day-innovation-showcase-palo-alto-organ-regeneration-startups-tickets-73172692493?aff=ebdssbdestsearch
Contacts:
For Valvublator:
Dr. Brett Burton, VP of R&D, bburton@leonhardtventures.com
Brian Hardy, Director of Marketing, brian@leonhardtventures.com
Phone: (424) 291-2133
web sites: www.valvublator.com
Leonhardt's Launchpads by Cal-X Stars Business Accelerator, Inc., 12655 W Jefferson Blvd, Los Angeles, CA 90066
Leonhardt's Launchpads Utah, Inc. 370 S, 300 E, Salt Lake City, UT 84111
Research Lab @ 2500 S State St. #224, Salt Lake City, UT 84115























